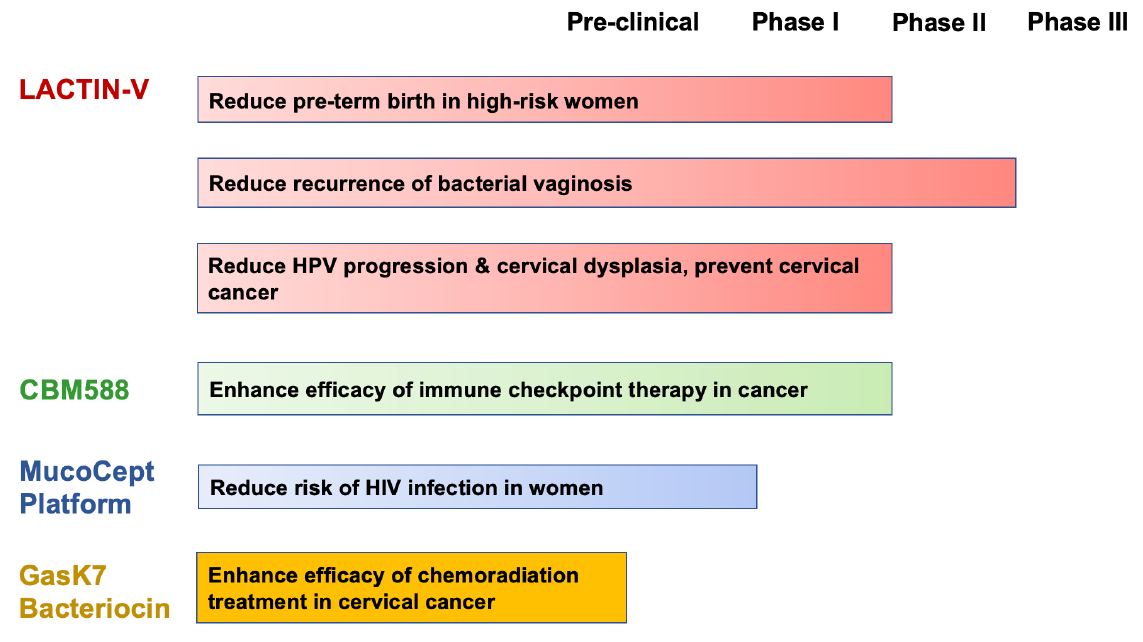
LACTIN-V
Osel’s LACTIN-V is a live biotherapeutic product addressing women’s health disorders. LACTIN-V is under development for preventing recurrent BV and preterm birth. It contains Lactobacillus crispatus CTV-05, a single strain of hydrogen peroxide-producing vaginal Lactobacillus that is part of the natural vaginal microbiome of many healthy women. It was selected for features that restore the vaginal microbiota, prevent urogenital infections and promote reproductive health. LACTIN-V targets clinical indications associated with dysbiosis of the vaginal microbiota where hydrogen peroxide-producing lactobacilli are depleted, such as bacterial vaginosis (BV). LACTIN-V significantly reduced recurrent BV in a Phase 2B clinical trial (NCT02766023). In addition, LACTIN-V reduced preterm birth (NCT03992534) in women at high risk by promoting the colonization of L. crispatus and reducing inflammatory mediators. LACTIN-V is delivered vaginally with a proprietary applicator.
Gassericin K7 Bacteriocin
Previously, we discovered a potent and selective inhibitor of L. iners. We demonstrated that the inhibitory activity consisted of two Class IIb two-peptide bacteriocins, gassericin K7A (GasK7A) and gassericin K7B (GasK7B). GasK7B was the more potent of the two bacteriocins, with nanomolar potency against L. iners. Independently, a causal role for L. iners in cervical dysplasia and cancer was recently elucidated by work from the Laboratory of Lauren Colbert at MD Anderson Cancer Center. They identified a tumor-specific pathotype of L. iners (oncogenic L. iners) that is significantly associated with recurrence of cervical cancer and poor survival of patients following chemoradiation therapy. These oncogenic L. iners strains induced cisplatin and radiation resistance in cervical cancer cells and organoids. We are collaborating with MD Anderson to synthesize the GasK7B bacteriocin and characterize its activity, selectivity, and in vitro safety to establish GasK7B as a potential drug candidate for the treatment of cervical cancer in conjunction with chemoradiation therapy.
CBM588
CBM588 is an orally administered Clostridium butyricum strain with a long history of clinical use and safety in all age groups in Japan. In Japan, CBM588 has primarily been used to treat infectious and noninfectious diarrheal disorders associated with dysbiosis of the human gastrointestinal microbiome. CBM588 inhibits certain enteric pathogens and supports colonic homeostasis by producing butyric acid. The strain also possesses novel immunomodulatory activities. CBM588 is being developed in the U.S. under an IND for prevention of antibiotic associated diarrhea, including Clostridium difficile infection. Three investigator-sponsored Phase 1 studies have been recently completed with CBM588, one in hematopoietic stem cell transplantation (NCT03922035) and two in advanced kidney cancer (NCT03829111) at the City of Hope Comprehensive Cancer Center. A follow up study with CBM588 in advanced or metastatic kidney cancer has recently been completed (NCT05122546).
MucoCept Technology Platform
Most viruses enter the body via mucosal surfaces. The natural microbiota at these sites, may neutralize some viral particles before infection of the host can occur. Using a proprietary technology, MucoCept harnesses the human microbiome and enhances it to further virus neutralization. In the current MucoCept platform technology, we have modified a commensal Lactobacillus to express a potent inhibitor of Human Immunodeficiency Virus type 1 (HIV-1). Proof of concept has been demonstrated in an animal model. An IND has been filed with FDA to conduct the first-in-human clinical study.
